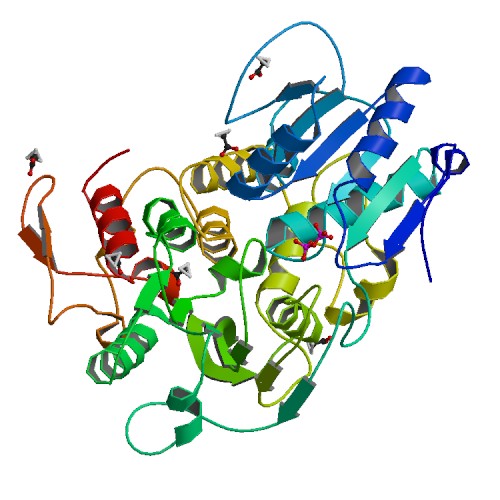
Structure of the NS3 helicase from Zika virus.
Publication Type:
Journal ArticleSource:
Nat Struct Mol Biol, Volume 23, Issue 8, p.752-4 (2016)Keywords:
Apoenzymes, Catalytic Domain, Crystallography, X-Ray, Models, Molecular, Protein Binding, Protein Conformation, alpha-Helical, RNA Helicases, Structural Homology, Protein, Viral Proteins, Zika VirusAbstract:
<p>Zika virus has emerged as a pathogen of major health concern. Here, we present a high-resolution (1.62-Å) crystal structure of the RNA helicase from the French Polynesia strain. The structure is similar to that of the RNA helicase from Dengue virus, with variability in the conformations of loops typically involved in binding ATP and RNA. We identify druggable 'hotspots' that are well suited for in silico and/or fragment-based high-throughput drug discovery.</p>
PDB:
5JRZ
Detector:
Q315
Beamline:
24-ID-E