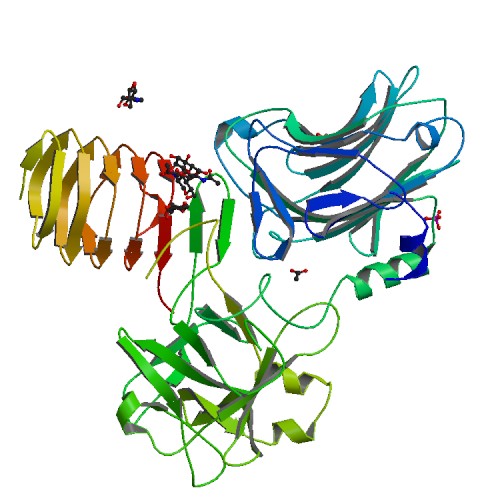
N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A.
Publication Type:
Journal ArticleSource:
Nat Struct Mol Biol, Volume 23, Issue 7, p.656-62 (2016)Keywords:
Amino Acid Sequence, Antibodies, Monoclonal, Antidotes, Binding Sites, Biological Transport, Botulinum Toxins, Type A, Cloning, Molecular, Clostridium botulinum, Crystallography, X-Ray, Escherichia coli, Gene Expression, Glycosylation, HEK293 Cells, Humans, Membrane Glycoproteins, Models, Molecular, Nerve Tissue Proteins, Protein Binding, Protein Domains, Protein Processing, Post-Translational, Protein Structure, Secondary, Recombinant Fusion ProteinsAbstract:
<p>Botulinum neurotoxin serotype A1 (BoNT/A1), a licensed drug widely used for medical and cosmetic applications, exerts its action by invading motoneurons. Here we report a 2.0-Å-resolution crystal structure of the BoNT/A1 receptor-binding domain in complex with its neuronal receptor, glycosylated human SV2C. We found that the neuronal tropism of BoNT/A1 requires recognition of both the peptide moiety and an N-linked glycan on SV2. This N-glycan-which is conserved in all SV2 isoforms across vertebrates-is essential for BoNT/A1 binding to neurons and for its potent neurotoxicity. The glycan-binding interface on SV2 is targeted by a human BoNT/A1-neutralizing antibody currently licensed as an antibotulism drug. Our studies reveal a new paradigm of host-pathogen interactions, in which pathogens exploit conserved host post-translational modifications, thereby achieving highly specific receptor binding while also tolerating genetic changes across multiple isoforms of receptors.</p>