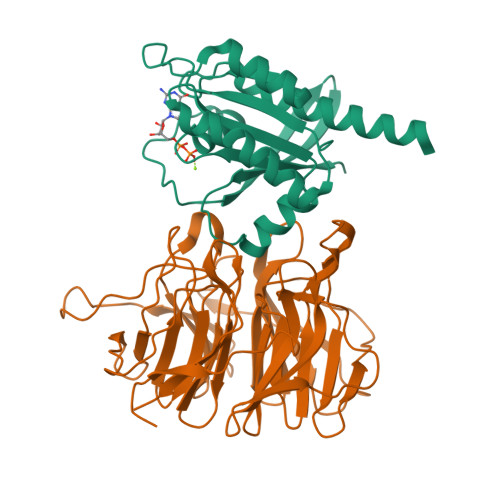
Structural basis for LZTR1 recognition of RAS GTPases for degradation.
Publication Type:
Journal ArticleSource:
Science, Volume 389, Issue 6765, p.1112-1117 (2025)Keywords:
Animals, Crystallography, X-Ray, Humans, Mice, Models, Molecular, Mutation, Protein Binding, Protein Domains, Proteolysis, Proto-Oncogene Proteins p21(ras), ras Proteins, Substrate Specificity, Transcription Factors, UbiquitinationAbstract:
<p>The RAS family of small guanosine triphosphatases (GTPases) are tightly regulated signaling molecules that are further modulated by ubiquitination and proteolysis. Leucine Zipper-like Transcription Regulator 1 (LZTR1), a substrate adapter of the Cullin-3 RING E3 ubiquitin ligase, binds specific RAS GTPases and promotes their ubiquitination and proteasomal degradation. We present structures of LZTR1 Kelch domains bound to RIT1, MRAS, and KRAS, revealing interfaces that govern RAS isoform selectivity and nucleotide specificity. Biochemical and structural analyses of disease-associated Kelch domain mutations revealed three types of alterations: impaired substrate interaction, loop destabilization, and blade-blade repulsion. In cellular and mouse models, mutations disrupting substrate binding phenocopied LZTR1 loss, underscoring its substrate specificity. These findings define RAS recognition mechanisms by LZTR1 and suggest a molecular glue strategy to degrade oncogenic KRAS.</p>