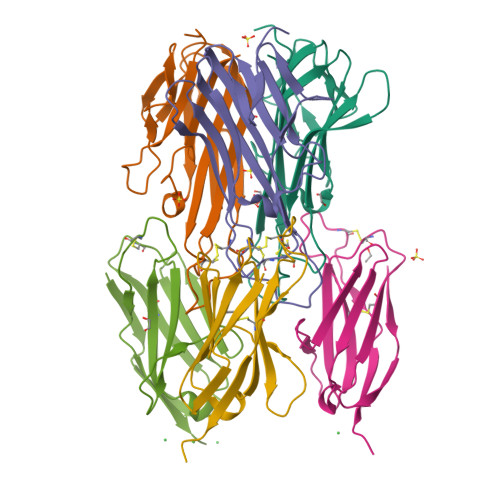
The structural basis for the selective antagonism of soluble TNF-alpha by shark variable new antigen receptors.
Publication Type:
Journal ArticleSource:
Nat Commun (2025)Abstract:
<p>The pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) is synthesized as transmembrane TNF-α (tmTNF-α) where proteolytic processing releases soluble TNF-α (sTNF-α). tmTNF-α can act as either a ligand by activating TNF receptors, or a receptor that transmits reverse (outside-to-inside) signalling after binding to native receptors. All TNF-α therapies bind tmTNF-α and induce reverse signalling which can result in immunosuppression leading to infection. We present crystal structures of two anti-TNF-α Variable New Antigen Receptors (VNARs) in complex with sTNF-α via two distinct epitopes. The VNAR-D1 recognizes an epitope that selectively engages sTNF-α while VNAR-C4 binds an epitope that partially overlaps with other biologic therapies. In activated CD4 T cells, our VNARs do not induce reverse signalling in contrast to currently available therapies. Our findings suggest that neutralization through a unique mechanism may lead to anti-TNF-α agents with an improved safety profile that will benefit high-risk patients.</p>