Molecular glues that facilitate RAS binding to PI3Kα promote glucose uptake without insulin.
Publication Type:
Journal ArticleSource:
Science, Volume 389, Issue 6758, p.402-408 (2025)Keywords:
Animals, Blood Glucose, Catalytic Domain, Class Ia Phosphatidylinositol 3-Kinase, Diabetes Mellitus, Experimental, Diabetes Mellitus, Type 2, Glucose, Humans, Hypoglycemic Agents, Insulin, Male, Protein Binding, ras Proteins, Rats, Rats, ZuckerAbstract:
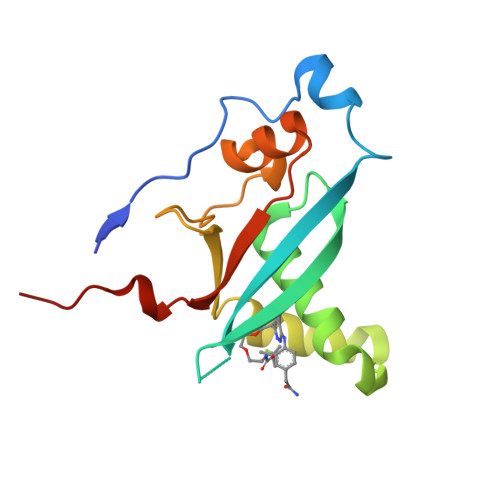
<p>While exploring strategies to control blood glucose concentrations in diabetes, we identified so-called molecular glues D223 and D927 that promote glucose uptake in the absence of insulin. They act by increasing the binding affinity of phosphoinositide 3-kinase α (PI3Kα) catalytic subunit p110α to canonical small guanosine triphosphatase RAS proteins and to RRAS, RRAS2, and MRAS by three orders of magnitude. The compounds bind to the RAS-binding domain of p110α, stabilizing the secondary structures of the PI3Kα in a RAS-binding conformation and forming direct interactions with RAS residues tyrosine-40 and arginine-41. In vivo, D927 mimicked the effects of insulin: It rapidly lowered blood glucose concentrations, enhanced glucose metabolism in normal and Zucker fatty rats, and improved hyperglycemia in models of type 1 and type 2 diabetes, even in insulin-deficient diabetic animals.</p>