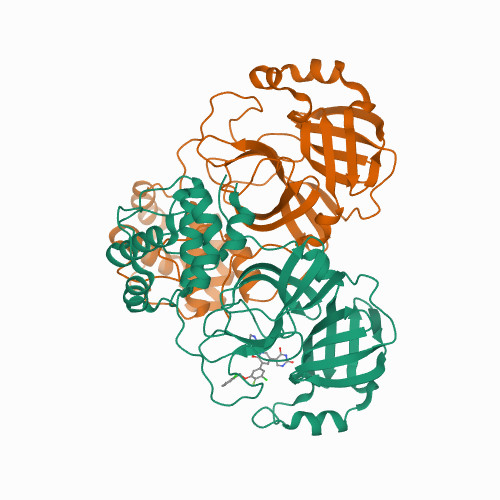
Structure-guided design of a perampanel-derived pharmacophore targeting the SARS-CoV-2 main protease.
Publication Type:
Journal ArticleSource:
Structure (2021)Abstract:
<p>There is a clinical need for direct-acting antivirals targeting SARS-CoV-2, the coronavirus responsible for the COVID-19 pandemic, to complement current therapeutic strategies. The main protease (M) is an attractive target for antiviral therapy. However, the vast majority of protease inhibitors described thus far are peptidomimetic and bind to the active-site cysteine via a covalent adduct, which is generally pharmacokinetically unfavorable. We have reported the optimization of an existing FDA-approved chemical scaffold, perampanel, to bind to and inhibit M noncovalently with ICs in the low-nanomolar range and ECs in the low-micromolar range. Here, we present nine crystal structures of M bound to a series of perampanel analogs, providing detailed structural insights into their mechanism of action and structure-activity relationship. These insights further reveal strategies for pursuing rational inhibitor design efforts in the context of considerable active-site flexibility and potential resistance mechanisms.</p>